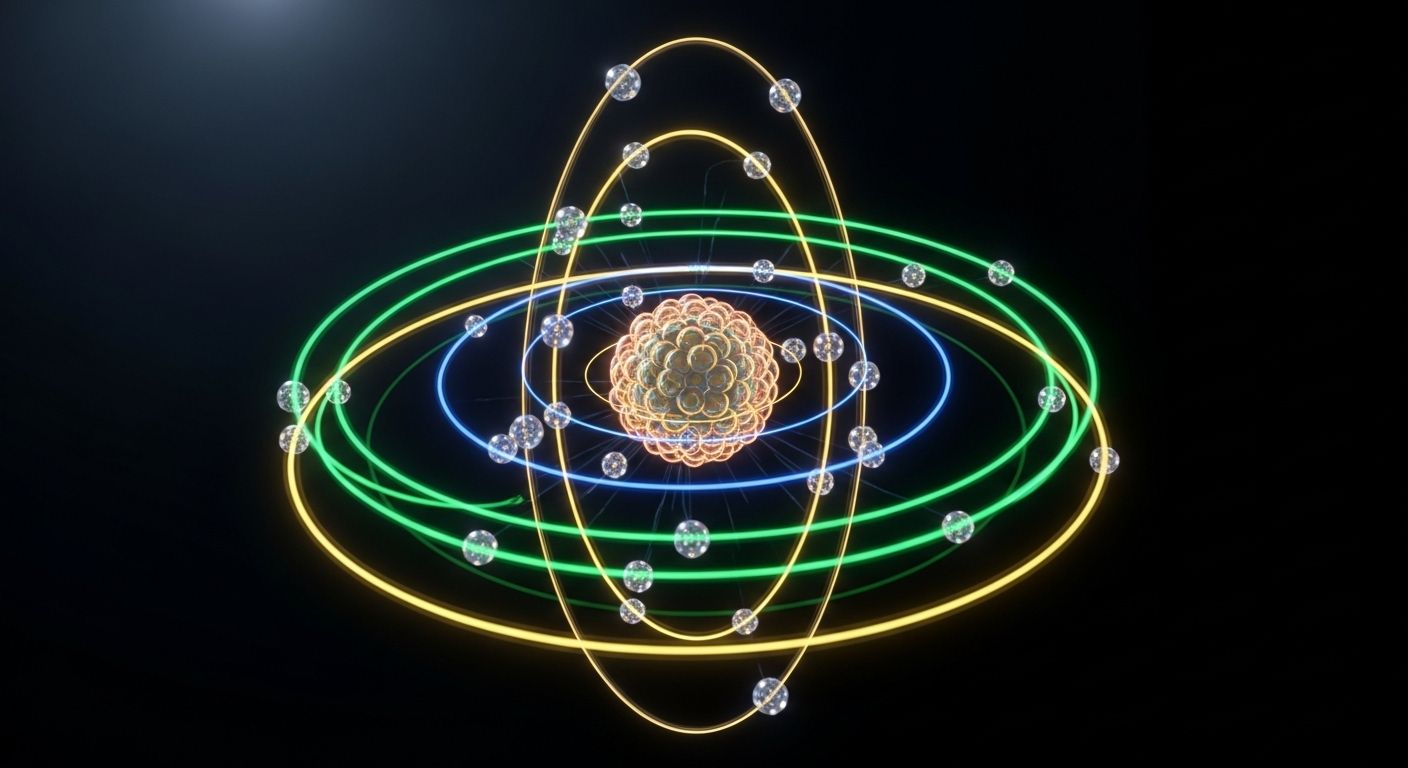
Electron Configuration and Orbitals is a foundational chemistry module that unlocks the blueprint of atomic behavior. Electrons don’t just orbit randomly—they follow precise rules that determine how atoms bond, react, and form the substances around us.
This lesson dives into the architecture of electron arrangement, revealing how quantum principles shape the periodic table and chemical interactions. Whether you’re a student, educator, or future scientist, this course equips you with the tools to decode atomic structure and predict elemental behavior.
🔬 By the end of this lesson, you’ll be able to:
This lesson is ideal for high school and college chemistry students, educators teaching atomic theory, and anyone curious about the quantum rules that govern chemical behavior.