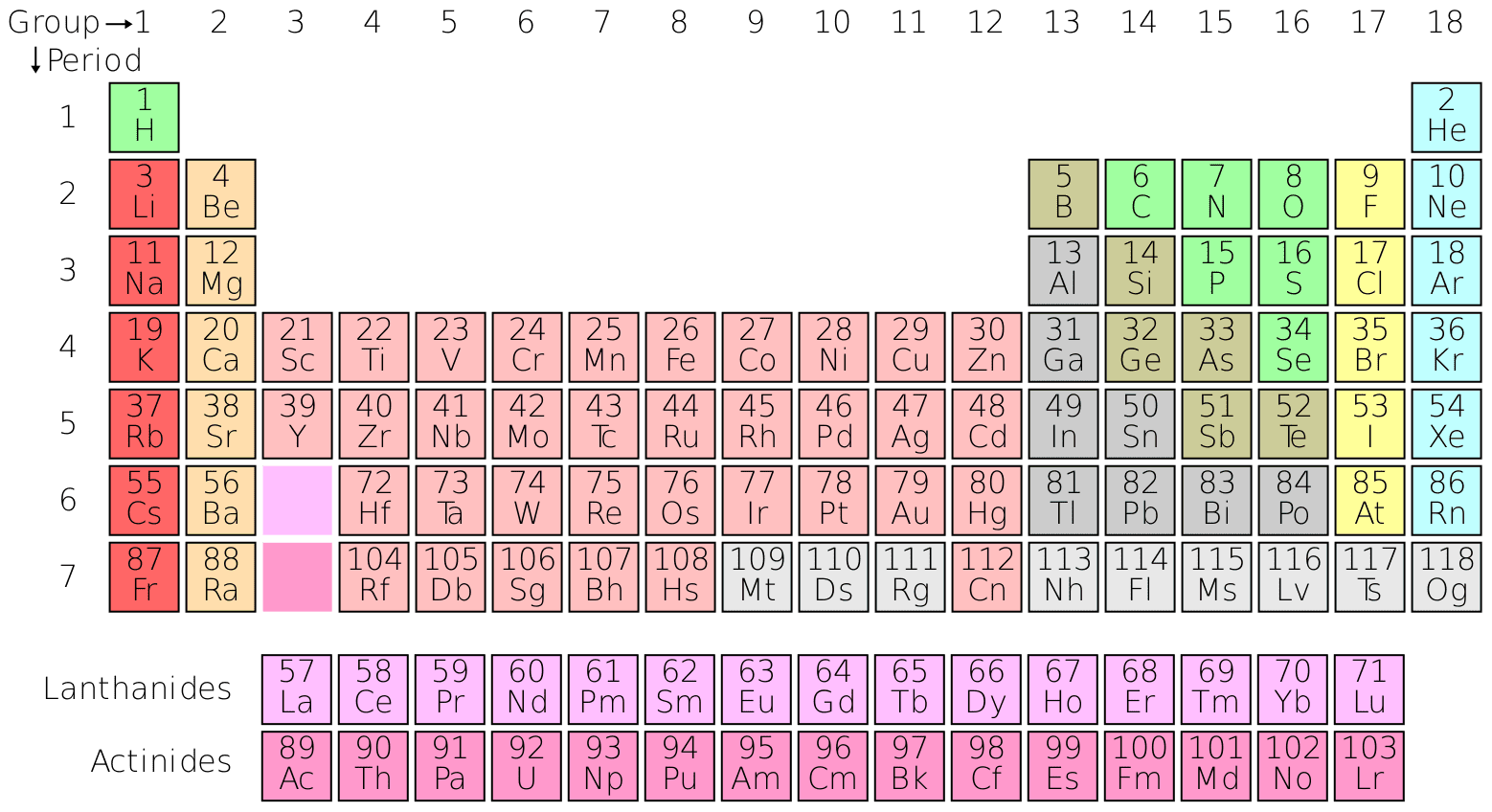
Exploring the Periodic Table is a foundational chemistry module that introduces one of the most powerful tools in science. The Periodic Table is more than just a chart—it’s a map of the building blocks of matter, revealing patterns, relationships, and predictions about how elements behave.
This lesson traces its historical development, explains its structure, and shows how it empowers scientists to understand and manipulate the material world. Whether you’re a student, educator, or curious explorer, this course will deepen your appreciation for the elegant logic behind chemical organization.
🧩 By the end of this lesson, you’ll be able to:
This lesson is ideal for students beginning their chemistry journey, educators teaching atomic theory, and anyone fascinated by the structure and logic of the natural world.